Intermittent Thoughts on Building Muscle: The Skeletal Muscle Hypertrophy 101 - Part 1: What is Hypertrophy?
I can already foresee that this is only the first in yet another series of posts. An introduction into the myocellular mechanisms that turn a normal teen like into a symbol of physical culture, or, to put it simply:
What actually is skeletal muscle hypertrophy?
Before I even try to answer this question let me remind you of something you have learned about "growth" in one of the previous installments of this series. In "Building Muscle Starts With Losing Weight" you have learned that one of the greatest fallacies of "classical" bulking, as in "eating everything that cannot escape your ravenous hunger for mass", is adipocyte hyperplasia. You may also remember that this increase in the number of fat cells occurs, when your existing fat stores are ready to burst and your body is in need of new storage capacities. Analogously, you would expect your muscle fibers to "hypertrophy" (from mechanical overload and constant nutrient abundance) until they are "ready to burst" and then divide and form new muscle fibers. (Un?)fortunately, myocytes are not adipocytes and thusly things are working somewhat different, here.
 |
| Figure 1: Overview over the three (?) pathways by which your skeletal muscles "grow". |
- Pathway A - hypertrophy via satellite cell recruitment and increases in the number of myonuclei per muscle fiber,
- Pathway B - hypertrophy via increases in myonuclear domain size within an existing muscle fiber, and
- Pathway C - hyperplasia, which would be the increase in muscle size by cell division and thusly an increase in the number of muscle fibers
I mean, you do not want "hypertrophy", but you want to get big and buffed, right?
Assuming that this is the case we should initially define "big and buffed" on a myofibrillar level by taking a look at how the muscles of the forerunners of physical culture actually look like - and I promise, what you will be learning today will, once again(!), go against conventional wisdom. Or would you have expected that bodybuilding is a sport that is characterized by a loss in highly glycolytic type IIb fibers and increases in both the intermediate type IIa, as well as the "endurance type" slow-twitch muscles? No? Well, then you should have a look at the data in figure 2:
 |
| Figure 2: Fiber composition of bodybuilders, recreational lifters, endurance rowers and sedentary control; determined via myosin heavy chain (MHC) isoform content of the triceps brachii muscle (data adapted from Jurimäe. 1997) |
It is interesting to note that Kraemer et al. (1995) have reported a lack of change in the area of fibres consisting predominantly of MHC type IIb proteins (i.e. FTb fibres) as a consequence of a 12-week resistance training programme. This suggests that a shift from MHC type IIb proteins to type IIa MHC isoforms may be a necessary prerequisite for FT fibre hypertrophy to occur. Consistent with this was the significant negative correlation (r = -0.67) between the percentage of MHC type IIb isoforms and arm circumference. Similarly, the smaller arm girth of the C group may have been partially due to the greater content of MHC type IIb isoforms in this group.Or put simply, the "strong" type IIb fibers have a very limited (if any) propensity for hypertrophy. So that, in order to maximize growth, it is necessary to trigger a shift towards the more "intermediate" type IIa fibers. If you take into consideration, how almost all bodybuilders got, where they are now, i.e. by a volume training approach, this is actually something you should have been able to infer simply from what has been and is still working for 99% of the trainees.
 |
| Figure 3: Intercorrelations between myosin heavy chain (MHC) isoforms and isoinertial (1-RM max), isometric (extension) and isokinetic (extension peak torque) strength indices (data adapted from Jurimäe. 1997) |
Skeletal muscle hyperplasia - yes or no? While there are a handful of studies which speak of hyperplastic
responses to stretch or other form artificial overload, many (if not
all) of them have been done on avian myofibers (Kelly. 1996),
which, due to their special make-up, make it a) very difficult to
distinguish between increasing overlap due to the longitudinal growth of
intrafascicularly terminating skeletal muscle fibers and "real"
hyperplasia and b) may not even translate to human beings. That's the reason, why I will disregard the issue of hyperplasia in the following discussion.
A very recent study by an international group of scientists from Sweden and the USA, may provide some insights, into why these fiber-transformations are necessary if you want to grow tree-trunk legs and sleeve-bursting arms. In this study, which was published on November 28, 2011, in the FASEB Journal (Qaisar. 2011), Rizwan Qaisar and his colleagues provide a detailed analysis of the the muscle fiber composition of mice who are either myostatin-negative or over-express the muscle building growth hormone IGF1 (we are talking about intra-muscular IGF1, here! More on that in future installments of the series). |
| Figure 4: Cross sectional area (CSA), number of mynuclei and myonuclear domain size of myostatin negative mice and mice overexpressing IGF1 relative to wild-type control (data calculated based on Qaisar. 2011) |
 |
| Image 2: The balloon metaphor of skeletal muscle hypertrophy. |
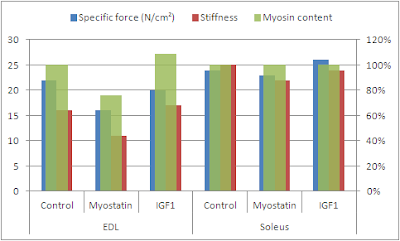 |
| Figure 5: Specific force, stiffness and myosin content (secondary axes) of EDL and soleus muscle in wild-type control, myostatin negative and IGF1 overexpressing mice (data based on Qaisar. 2011) |
 |
| Figure 6: Domain sizes of EDL and soleus muscle fibers in wild-type control, myostatin negative and IGF1 overexpressing mice (data based on Qaisar. 2011) |
Muscle hypertrophy = increases in myonuclear number & domain size
Healthy muscle growth, that is the intermittent take-away of this installment of the Intermittent Thoughts on building muscle, is thusly a direct result of "hypertrophy", as it is commonly associated with increased protein synthesis (and decreased or constant protein breakdown) and the subsequent expansions of individual myonuclear domains and the recruitement of satellite cells, which will then form new myonuclei.
As you may have noticed from the increasing amount of typos, of which I have probably overlooked 50% (sorry for that), my Sunday time-budget is already exhausted, so that I will have to postpone the discussion of what triggers these processes to the next installment. I do yet hope that the stuff you learned today provides enough food for thought to get you through the week ;-)



